Electronics
| With strontium titanate, a stable two-dimensional electron gas has been created by researchers. The electrons in this gas can occupy very different quantum states with very different properties. This new material is a remarkable new instrument for designing electronics and possibly using exotic material effects such as superconductivity. |
Usually, microelectronic devices are made of silicon or similar semiconductors. Recently, the electronic properties of metal oxides have become quite interesting. These materials are more complex, yet offer a broader range of possibilities to tune their properties. An important breakthrough has now been achieved at the Vienna University of Technology: a two dimensional electron gas was created in strontium titanate. In a thin layer just below the surface electrons can move freely and occupy different quantum states.
The research has been published in the journal Proceedings of the National Academy of Sciences (PNAS).
Strontium titanate is not only a potential future alternative to standard semiconductors, it could also exhibit interesting phenomena, such as superconductivity, thermoelectricity or magnetic effects that do not occur in the materials that are used for today’s electronic devices.
This project closely links theoretical calculations and experiments. Zhiming Wang from Professor Ulrike Diebold’s research team was the leading researcher; some of the experimental work was done at the synchrotron BESSY in Berlin. Zhicheng Zhong from Professor Karsten Held’s group studied the material in computer simulations.
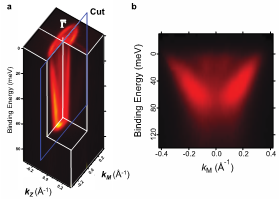 |
| Image Source - Wang et al. |
| Related articles |
Something remarkable happens when the material is irradiated with high-energy electromagnetic waves: “The radiation can remove oxygen atoms from the surface”, Diebold explains. Then other oxygen atoms from within the bulk of the material move up to the surface. Inside the material, an oxygen deficiency builds up, as well a surplus of electrons.
“These electrons, located in a two dimensional layer very close to the surface, can move freely. We call this an electron gas”, says Karsten Held. There has already been some evidence of two dimensional electron gases in similar materials, but until now the creation of a stable, durable electron gas at a surface has been impossible. The properties of the electrons in the gas can be finely tuned. Depending on the intensity of the radiation, the number of electrons varies. By adding different atoms, the electronic properties can also be changed.
“In solid state physics, the so-called band structure of a material is very important. It describes the relationship between the energy and the momentum of the electrons. The remarkable thing about our surface is that it shows completely different kinds of band structures, depending on the quantum state of the electron”, says Held.
The electron gas in the new material exhibits a multitude of different electronic structures. Some of them could very well be suitable for producing interesting magnetic effects or superconductivity. The promising properties of strontium titanate will now be further investigated. The researchers hope that, by applying external electric fields or by placing additional metal atoms on the surface, the new material could reveal a few more of its secrets.
SOURCE Vienna University of Technology
| By 33rd Square | Subscribe to 33rd Square |







0 comments:
Post a Comment